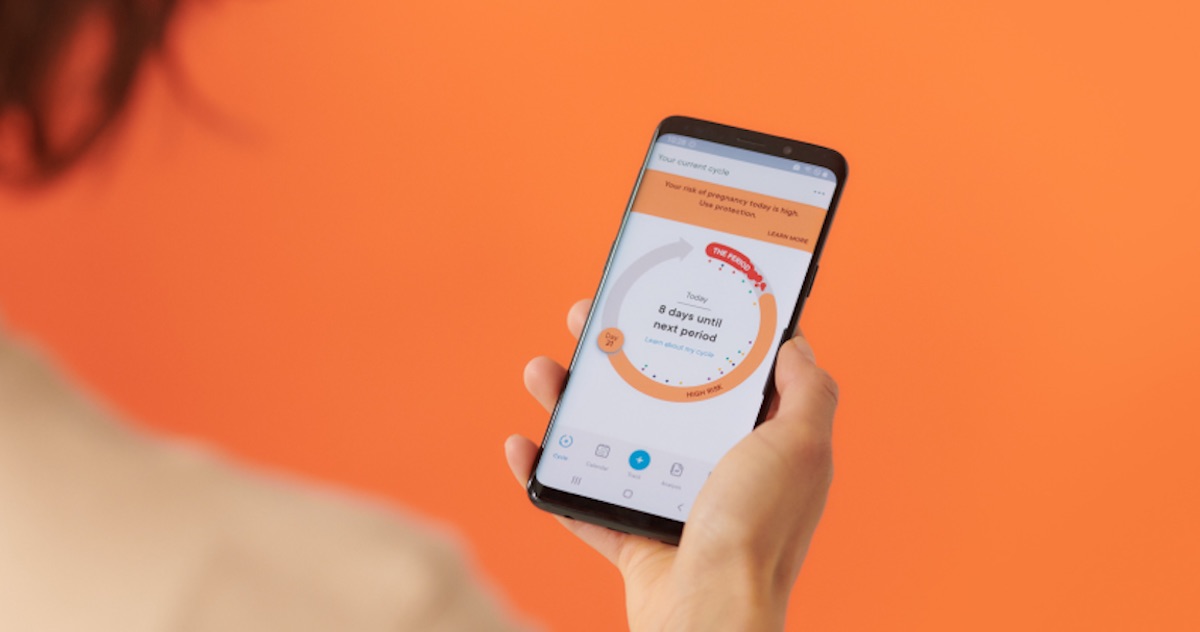
Clue, an early pioneer in the femtech category with a well-regarded period-tracking app that’s used by around 13 million people, is getting ready to launch a digital contraceptive which will offer users a statistical prediction of ovulation as a birth control tool.
A US launch of Clue Birth Control is slated for “this year.” The team won’t be more specific on the date yet.
Pricing will be “premium” — but they’re also keeping the exact cost under wraps for now.
The Berlin-based company announced that they’ve gained FDA clearance for the forthcoming product, clearing the way for a US launch in 2021.
Europe is the next region on their launch list but the necessary regulatory clearances mean the roll out will be gradual, going market by market.
Clue is not the first to market with an app offering a statistical prediction of fertility but unlike first mover, Natural Cycles — which gained FCA clearance back in 2018 — its product, Clue Birth Control, is being billed as “all-digital”; aka it will work without the user needing to take their temperature daily.
Clue’s algorithmic prediction is based on Bayesian modelling — with the app displaying a high risk window across a number of days of the user’s cycle, during which having unprotected intercourse could result in the woman getting pregnant. It shows a window of low risk when the opposite is true.
The length of these windows is likely to change as the user inputs more data, with the risk-window starting out longer and typically shrinking as the app becomes more personalized to their individual cycle data.